| ULTRAMAFITE - MAFITE |
In the field, ultramafic rocks are mostly seen in the basal sections of ophiolites and as exhumed pieces of the upper mantle. Here''s a famous outcrop at Helgehornvatnet in Norway |
The layering is formed by different proportions of olivine, opx, cpx, and garnet-Helgehornvatnet in Norway |
Helgehornvatnet in Norway - Which you can see nicely in this closeup |
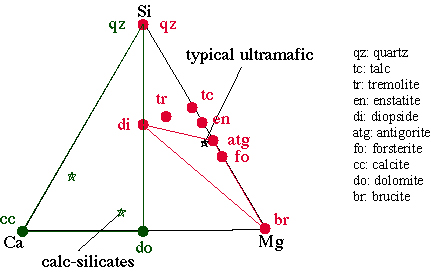
To understand the petrogenesis of ultramafic rocks using the CaO-MgO-SiO system, we plot the phases using molar proportions on a triangle with one component at each apex |
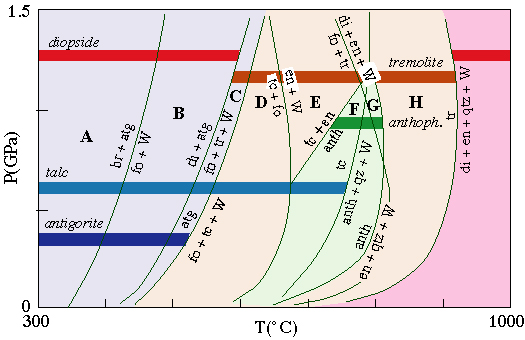
Reactions in the CMS(H) system at low pressures--the model system for peridotite |
anthophyllite is stable only at amphibolite-facies conditions, and only to pressures of ~1.2 GPa, rendering it the only useful indicator of pressure in this group of minerals |
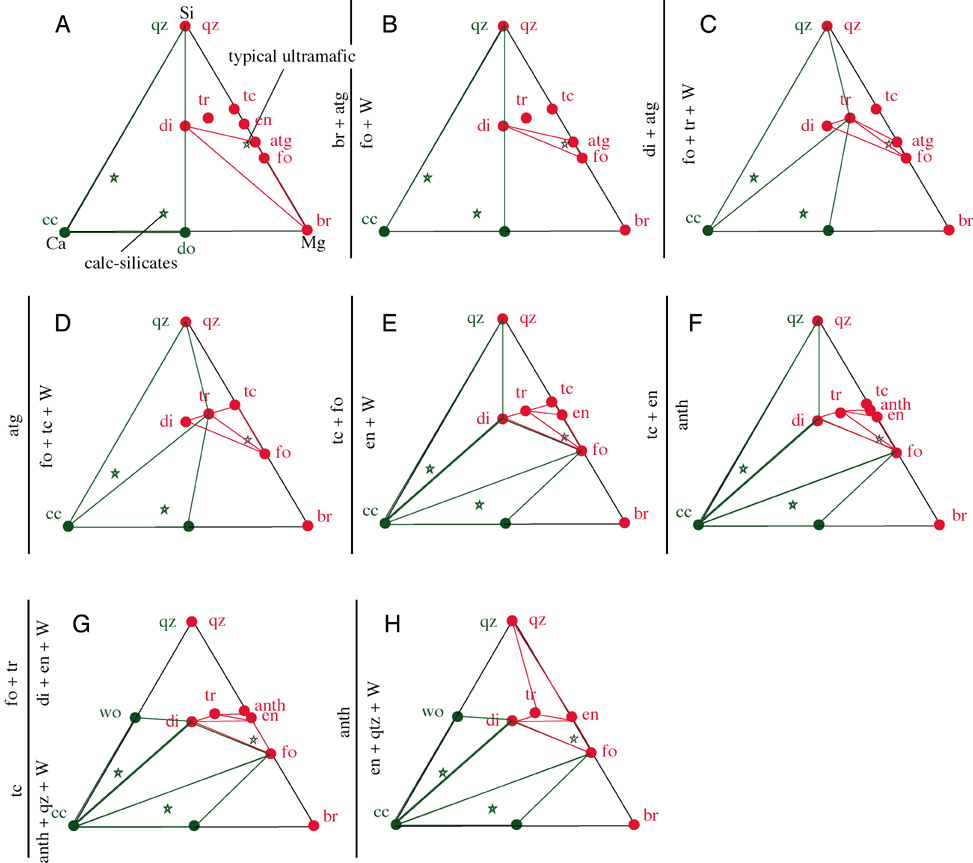
Below are phase diagrams showing the stability fields of minerals in various bulk compositions of ultramafic rocks |
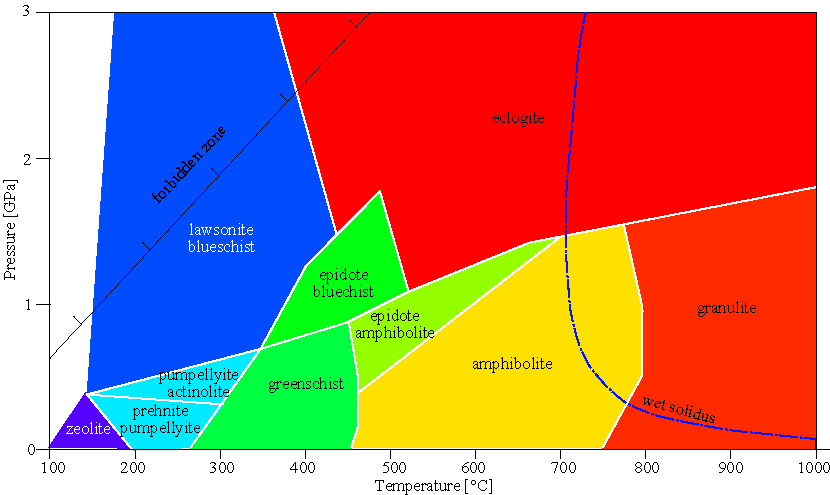
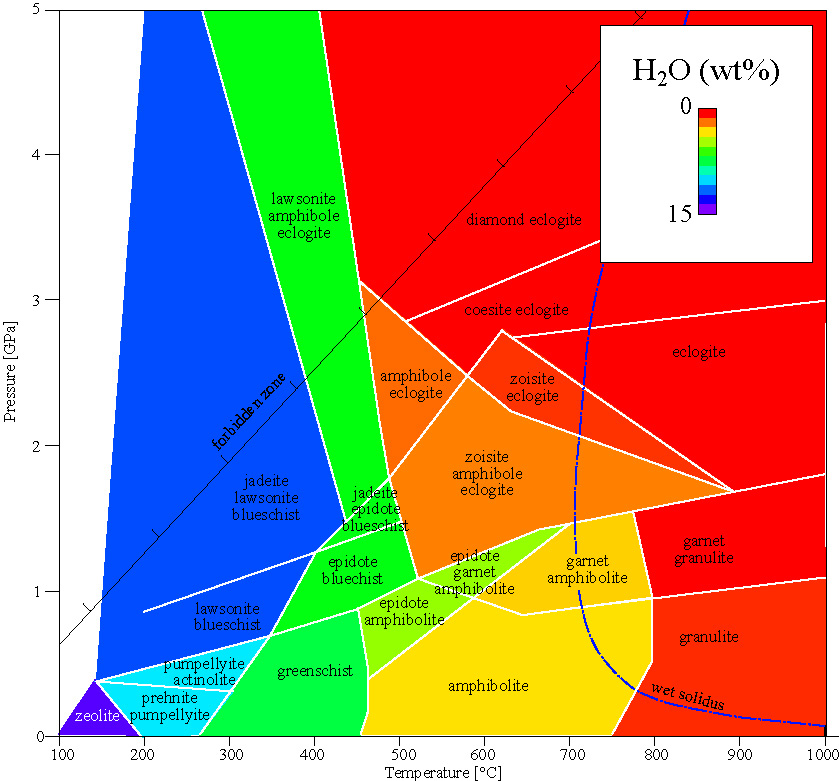
Mafic facies |
Mafite: facies in complex |
Source: http://www.geol.ucsb.edu/faculty/hacker/geo102C/lectures/part5.html
http://www.geol.ucsb.edu/faculty/hacker/geo102C/lectures/part6.html
The three-component system CMS (Ca-Mg-Si): Ultramafic Rocks
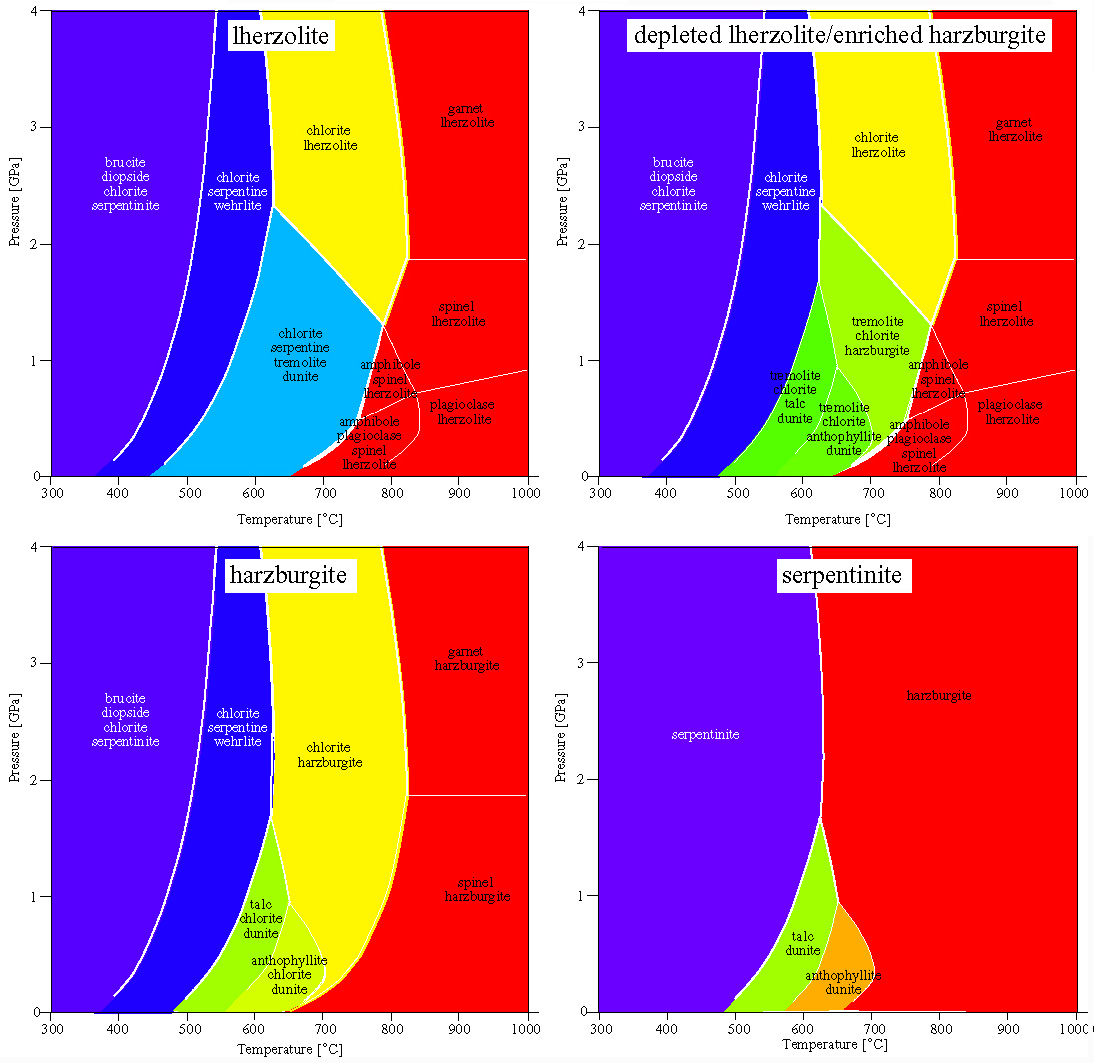
The upper mantle is composed of ultramafic rocks (peridotites), different proportions of olivine (forsterite to fayalite) + orthopyroxene (enstatite to ferrosilite) + clinopyroxene (diopside to hedenbergite). Enriched upper mantle is lherzolite: olivine + opx + cpx. Extraction of basalt via melting leads to loss of the cpx and the production of an olivine + opx residuum called harzburgite. Metamorphism of harzburgites can conveniently be modeled using Mg-Si-O-H. Modeling of meta-lherzolites requires the addition of Ca, which can be done with three components as CaO-MgO-SiO, assuming excess H2O. Peridotites also contain an aluminous phase, either plagioclase, spinel, or garnet.
To understand the petrogenesis of ultramafic rocks using the CaO-MgO-SiO system, we plot the phases using molar proportions on a triangle with one component at each apex:
forsterite Mg2SiO4
enstatite MgSiO3
tremolite Ca2Mg5Si8O22(OH)2
forsterite olivine (Mg,Fe)2SiO4)
serpentine (the three polymorphs are chrysotile, lizardite, antigorite) contains (Mg,Fe)SiO(OH)
anthophyllite Mg7Si8O22(OH)2
talc Mg6Si8O20(OH)2
PT Region--Divariant Field
if there are 2 degrees of freedom (P and T), 2 + P=3 + 2, P=3; there are three phases in a divariant field
these three phases define a triangle on the CMS diagram
bulk composition lies within triangle
a different bulk composition defines a different 3-phase triangle at the same P&T
Mineral Reaction--Univariant Line
if there is 1 degree of freedom (P or T), 1 + P=3 + 2, P=4; there are four phases along a univariant line--a reaction
the four phases create crossing tie lines
bulk composition lies within the intersection of the two 3-phase triangles
Invariant Point
if there are 0 degrees of freedom, 0 + P=3 + 2, P=5; there are five phases at an invariant point
Reactions in the CMS(H) system at low pressures--the model system for peridotite.
antigorite is stable to ~400░C, and is equivalent to greenschist-facies conditions
tremolite appears at conditions close to where antigorite disappears, and indicates amphibolite facies
talc is stable to much higher temperatures of ~750░C, and breaks down at granulite-facies conditions
diopside is stable at both low temperatures and high temperatures, separated by an interval in which tremolite is stable instead
anthophyllite is stable only at amphibolite-facies conditions, and only to pressures of ~1.2 GPa, rendering it the only useful indicator of pressure in special group of minerals.
Most ultramafic rocks span a very restricted range of bulk compositions and thus their constituent phases are also very restricted in composition. Because of this, ignoring Fe to calculate the phase relations is not a big simplification. This also means that the compositions of minerals cannot change much within their stability fields, the positions of the reactions do not move around very much with mineral composition, and the reactions are essentially discontinuous reactions.
MAFIC ROCKS
Solid Solutions and Exchange Vectors
Some minerals (e.g., quartz) have constant composition. However, most minerals are solid solutions that undergo compositional change in response to PTX (pressure, temperature, composition) changes. Garnet is an example of a relatively simple solid solution, with substitution occurring on only one site:
almandine Fe3Al2Si3O12
pyrope Mg3Al2Si3O12
grossular Ca3Al2Si3O12
spessartine Mn3Al2Si3O12
mineral end members exchange vector
olivine forsterite Mg2SiO4 <Ś> fayalite Fe2SiO4 Fe2+ <Ś> Mg2+
plagioclase albite NaAlSi3O8 <Ś> anorthite CaAl2Si2O8 Na+ Si4+ <Ś> Ca2+ Al3+
amphibole tremolite Ca2Mg5Si8O22(OH)2 <Ś> tschermakite Ca2(Mg3Al2)(Al2Si6)O22(OH)2 VIMg2+ IVSi4+ <Ś>VIAl3+ IVAl3+ (tschermak exchange)
mica muscovite KAl2(AlSi3)O10(OH)2 <Ś> phengite KMgAlSi4O10(OH)2 VIAl3+ IVAl3+ <Ś> VIMg2+ IVSi4+(inverse tschermak exchange)
Metamorphic Facies
Metamorphic facies are defined for metamafic rocks.
It is a fact of the universe that the minerals in mafic rocks tend to have broad ranges in solid solution. This means that
mafic rocks have fewer minerals than pelitic rocks
a given mineral in a mafic rock is stable over a broad PT range by virtue of its ability to shift composition in response to changes in P and T
reactions in mafic rocks are continuous reactions that span a range of PT space over which the minerals change in composition and abundance
facies minerals notes
basalt and gabbro protoliths plagioclase NaAlSi3O8-CaAl2Si2O8 + pyroxene CaSiO3-MgSiO3-FeSiO3 .
zeolite facies common zeolites: laumontite, wairakite, analcime (Na,Ca)AlSiO(OH)x zeolites: channel structure makes them useful for engineering
common textures: in cracks, alteration of feldspar or glass
greenschist facies albite + epidote + actinolite + chlorite + quartz
albite NaAlSi3O8 takes up Na, Al, Si
epidote CaAlSiO(OH)x takes up Ca
actinolite Ca2(Fe,Mg)5Si8O22(OH)2 takes up Fe, Mg
chlorite (Mg,Fe)(AlSi)O(OH)x .
albite-epidote amphibolite facies albite + epidote + hornblende + quartz
albite NaAlSi3O8 takes up Na, Al, Si
epidote CaAlSiO(OH)x takes up Ca
hornblende (K,Na)(Ca,Fe,Mg)(AlSi)O22(OH)2 modal proportion of albite decreases, Na enters amphibole
modal proportion of chlorite decreases, Fe + Mg enters amphibole
amphibolite facies plagioclase + hornblende + quartz epidote replaced by intermediate plagioclase
remainder of elements in hornblende
granulite facies orthopyroxene + clinopyroxene + plagioclase + quartz must have 2 pyroxenes; can also have hornblende or garnet
blueschist facies glaucophane Na2Mg3Al2Si8O22(OH)2
lawsonite or epidote CaAlSiO(OH)x high P, low T
eclogite facies omphacite (Na,Al,Mg,Fe)Si2O6
garnet (Ca,Mg,Fe,Mn)3Al2Si3O12 high P, high T
coesite-eclogite facies coesite SiO2
diamond C
majorite Mg4Si4O12 pressures >2.5 GPa
Facies Series
A series of facies at low, medium, or high pressure. Metamorphic rocks formed by crustal extension are characterized by a low-pressure facies series. Metamorphic rocks formed during continental collision are characterized by a medium-pressure facies series. Subduction-zone metamorphic rocks are characterized by a high-pressure facies series. facies series tectonic setting minerals present minerals absent
low P (Buchan) crustal extension andalusite, sillimanite, cordierite staurolite, kyanite
crustal thickening medium P (Barrovian) kyanite, sillimanite, staurolite cordierite
subduction high P (Franciscan) kyanite staurolite