| Physical Processes that Cause Metamorphism |
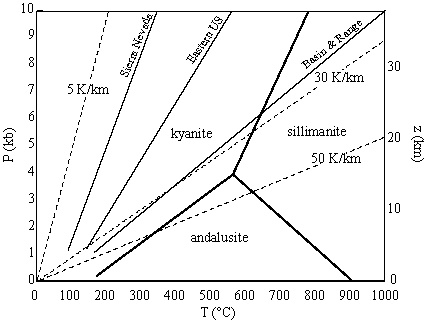
Geotherms |
|
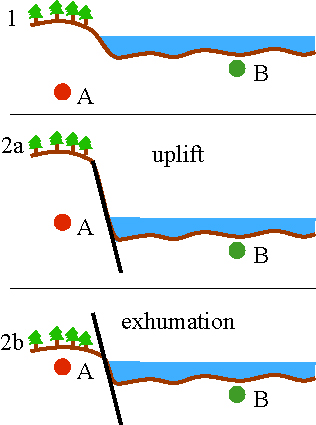
Uplift-exhumation |
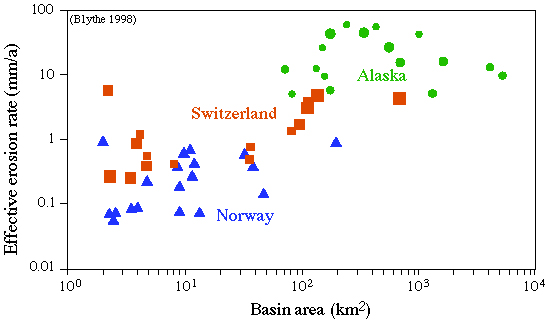
Erozion rates |
|
|
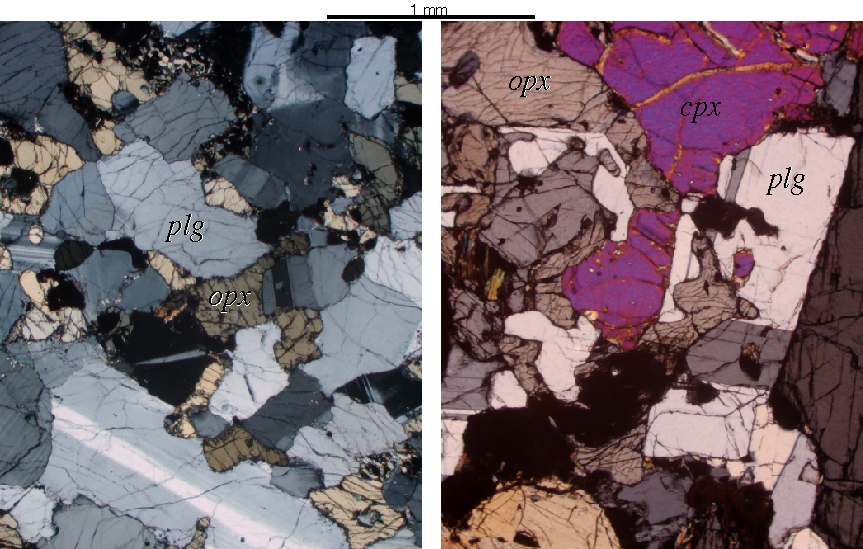
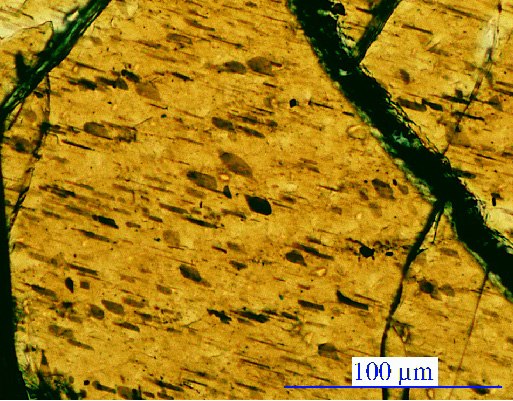
Granulites look a lot like igneous rocks |
Eclogites consist mostly of two minerals: garnet and Na-rich clinopyroxene known as omphacite |
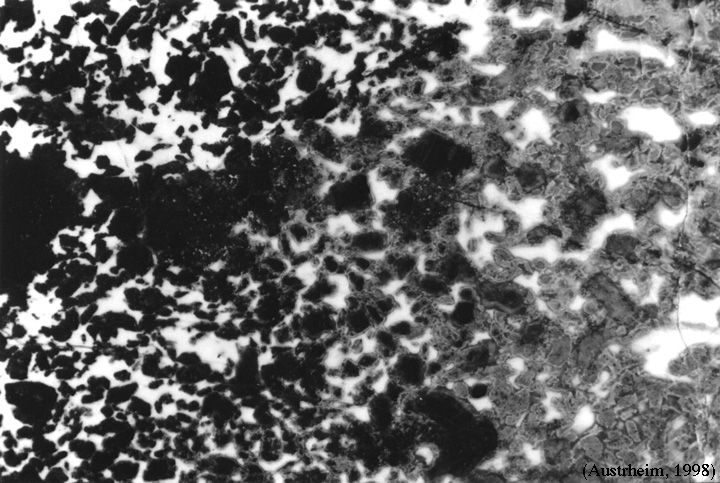
Norway show partial transformation of granulites to eclogites |
This transition takes place along a clear reaction front whose progress is mediated by fluid flow |
This transition takes place along a clear reaction front whose progress is mediated by fluid flow |
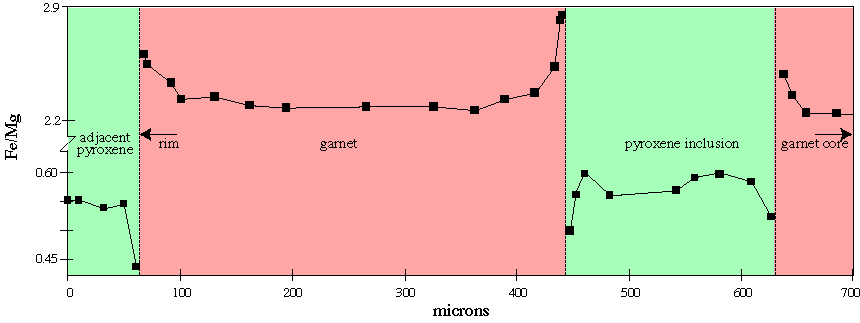
At the grain scale, disequilibrium is often characterized by zoning |
Transformation: Neoblasts can be observed once they have grown to a sufficient size |
The growth of one phase into another depends on factors including the Gibbs free energy change of the reaction, interfacial free energy, strain free energy, and temperature. Growth mechanisms can be studied in considerable detail with transmission-electron microscopy |
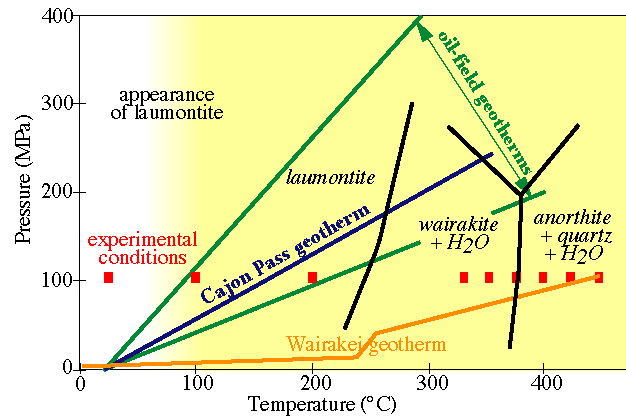
As a specific example of kinetic experiments, let''s consider the transformation of laumontite (a zeolite) to wairakite (another zeolite) + quartz + H2O |
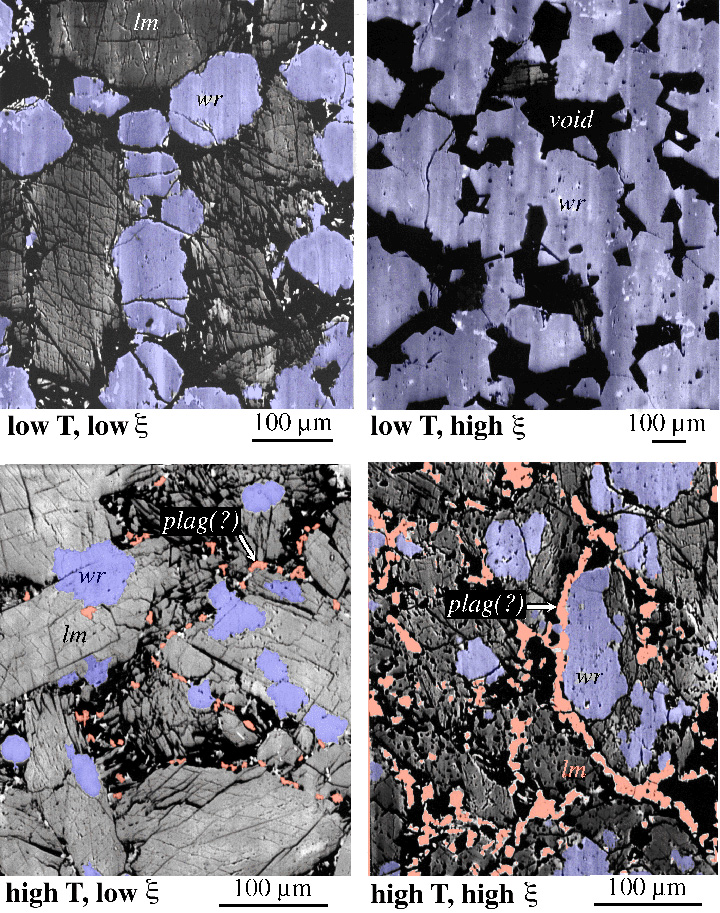
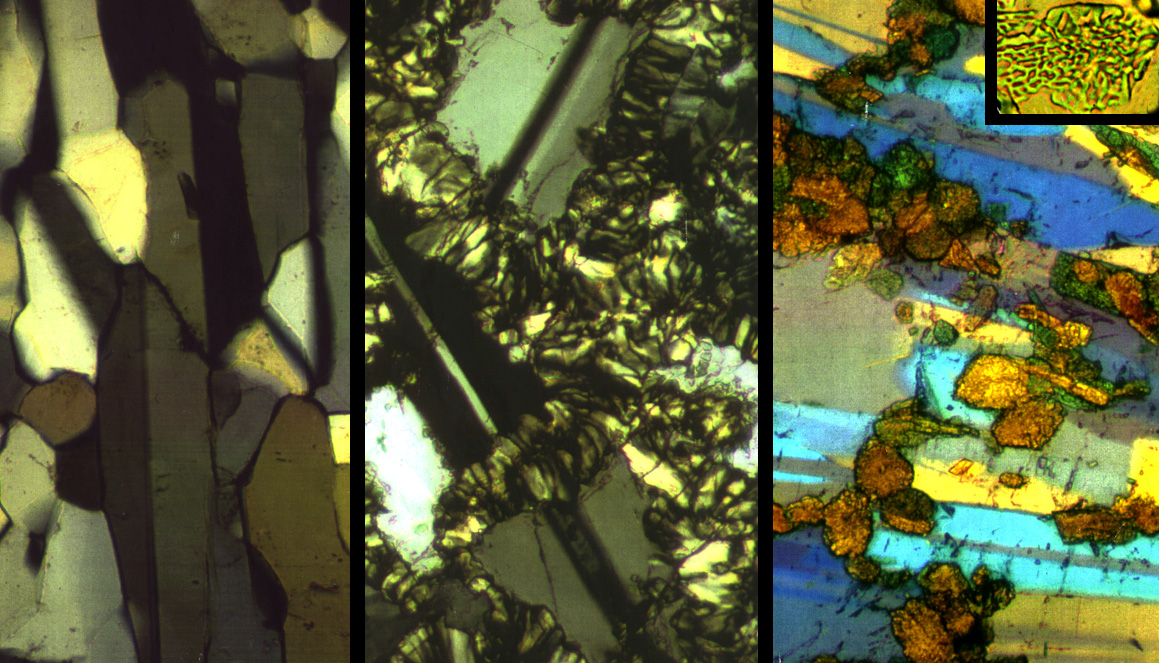
Different textures develop at low and high temperatures |
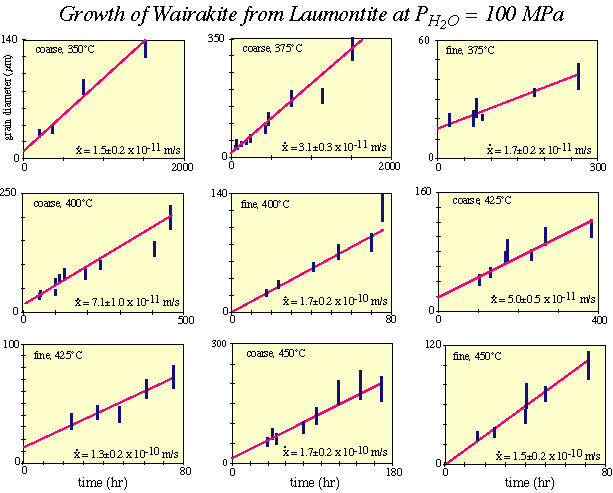
The growth rate in each experiment is determined by measuring the sizes of neoblasts |
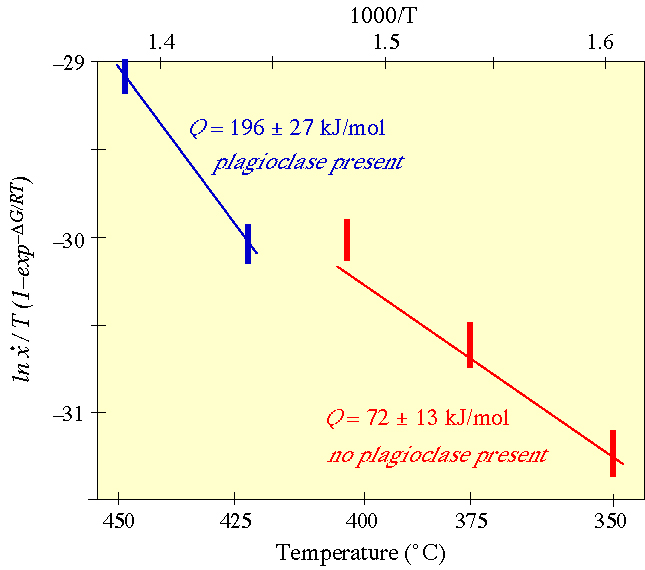
The apparent activation enthalpy of the growth rate can be determined from an Arrhenius plot |
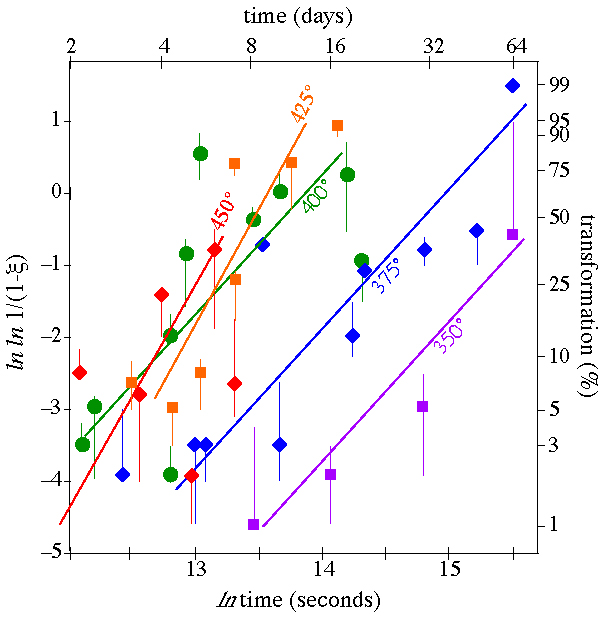
The rate of the entire transformation is a result of nucleation and growth |
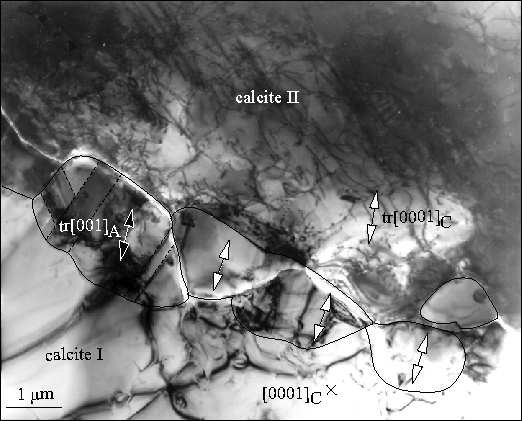
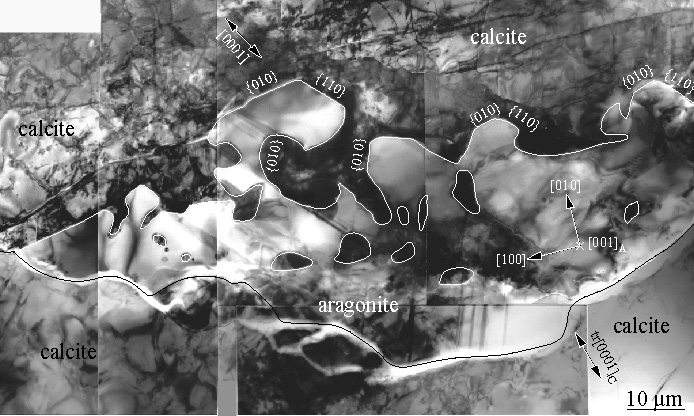
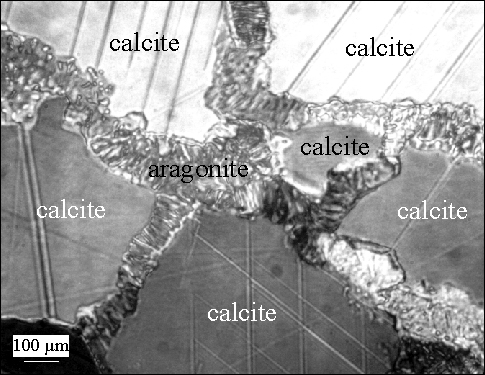
Example2: the transformation of calcite to aragonite.Small amounts of transformation are characterized by growth of aragonite along all calcite grain boundaries |
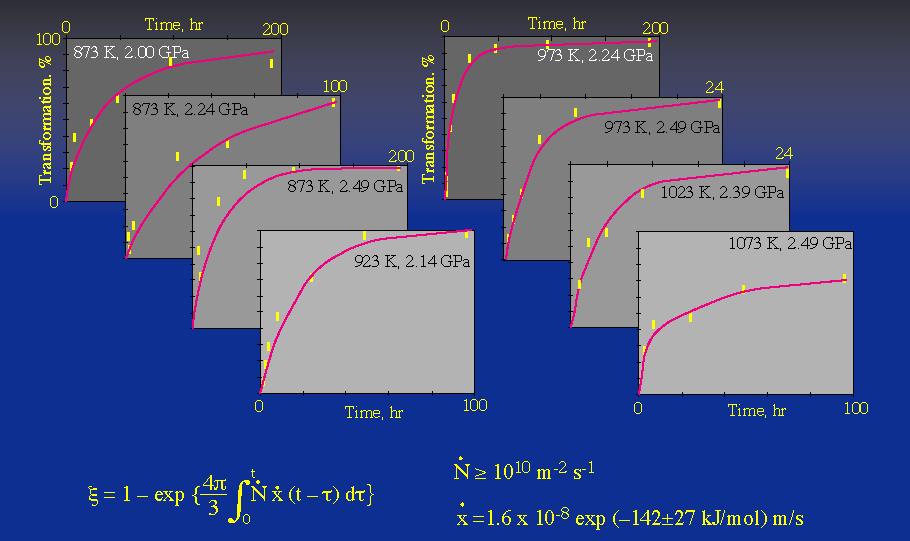
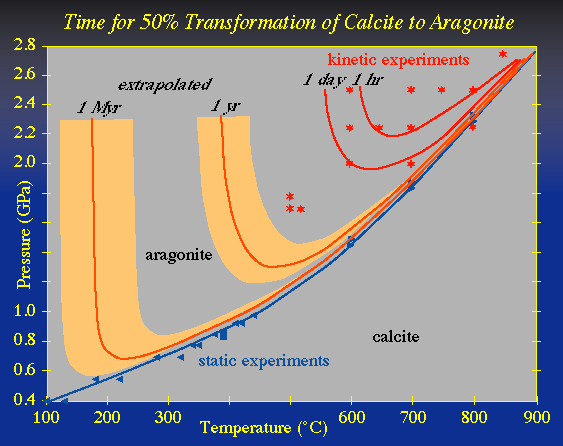
Measurements of the complete transformation rate as a function of pressure and temperature can be fit to an equation that includes nucleation and growth rate |
Under favorable circumstances, such data may be extrapolated to conditions in the Earth |
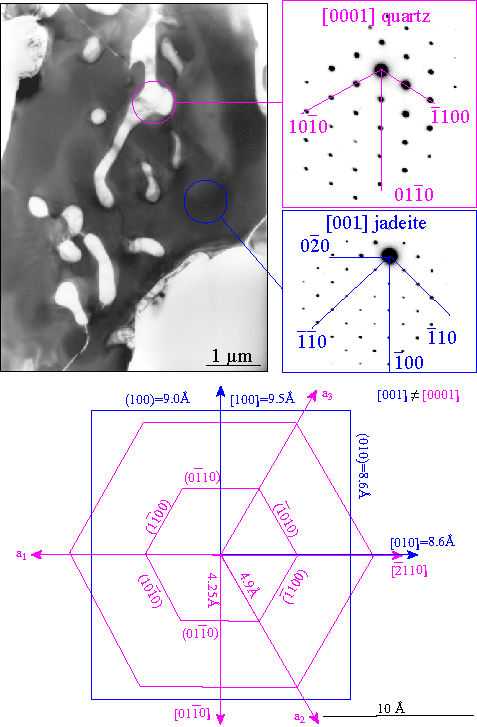
Textures are often a strong function of H2O content. The transformation of albite (left panel) to jadeite + quartz, for example, changes from discrete, rare nuclei in the presence of H2O (right panel), and many widespread nuclei in the absence of H2O (middle panel) |
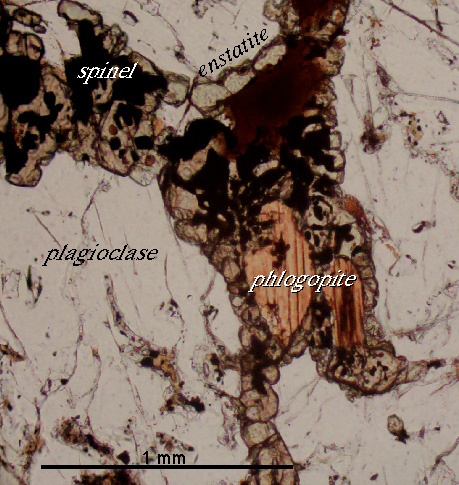
Here''s the texture produced by the temperature-induced breakdown of biotite to spinel + orthopyroxene |
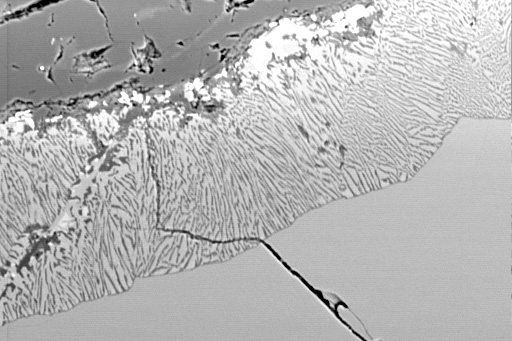
Symplectites result from decomposition reactions occurring under conditions where new crystals form and the diffusion rate is slower than the grain-boundary migration rate. Although product phases often have topotactic relationships with each other and/or the host phase, the shapes of the reaction products are often complex and controlled by the habit of the host phase |
Characteristic of low H2O activities and thus slow grain-boundary diffusion are symplectites. This is a garnet-breakdown symplectite, often called kelyphite |
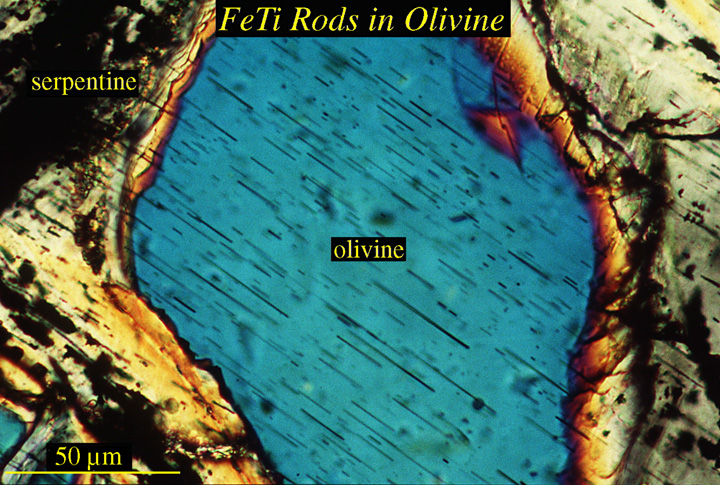
New minerals can also exsolve from host phases. In this case, while topotactic relationships are also typical, the shapes of the reaction products are often simple and controlled by the crystallography of the host phase. Some lherzolites contain olivine with rods of FeTiO3 |
Orogenic lherzolites have olivines with exsolved plates of magnetite |
Plates of ilmenite exsolve high-pressure rutiles |
Source: http://www.geol.ucsb.edu/faculty/hacker/geo102C/lectures/part3.html
http://www.geol.ucsb.edu/faculty/hacker/geo102C/lectures/part10.html
TEMPERATURE
heat, q, is energy (calories or Joules) in the form of molecular and atomic vibrations. When hotter, materials undergo more rapid vibrations.
the change in the amount of heat that a material contains is related to its heat capacity CP and the temperature change dT:
dq = CP dT
(CP is the heat capacity at constant pressure; ~1 kJ/kgK for rocks). For example, the amount of heat required to heat 1 m3 of rock of density 3000 kg/m3 20░C is: 1 kJ/kgK * 3000 kg * 20░C = 60,000 kJ. Recall that your suggested daily intake of energy is 2000 "calories", (actually kilocalories), which is equivalent to 8370 kJ (1 cal = 4.184 J).
(geo)thermal gradient:
change in temperature T with depth z
= 5-10 K/km in cold subduction zones
= 20 K/km in stable cratons
= 50 K/km in rifts and magmatic arcs
production of heat in the Earth
The heat of the Earth was produced by its early accretion and continues to be produced by the crystallization of the inner core from the outer core (remember that melting requires heat, so crystallization must give off heat), and the radioactive decay of unstable isotopes or radionuclides (chiefly the decay schemes 235U Ś> 207Pb, 238U Ś> 206Pb, 40K Ś> 40Ar, and 232Th Ś> 208Pb)
Three processes transfer heat in the Earth:
conduction: the transfer of heat by molecular vibration. Fourier''s law states that the heat flux q (flow of heat per unit area and per unit time) is: q=kdT/dz, where k is thermal conductivity (~3 J/mKs or ~3 W/mK). For example, in a thermal gradient of 20 K/km, the heat flow that results is 3 W/mK * 0.02 K/m = 60 mW/m2
A rough measure of the distance that a thermal pulse can travel in a specific time (or the amount of time required for a thermal pulse to travel a certain distance) is given by the characteristic diffusion distance u=SQRT(Kt), where K is the thermal diffusivity (~1E-6 m2/s) and t is time (s). For example, in 1 Ma (3E13 s), the characteristic diffusion distance is SQRT(1E-6 m2/s * 3E13) = ~5 km.
convection: the transfer of heat by buoyancy-driven circulation
Example: as rock in the lower mantle is heated, its density decreases because of thermal expansion, and it rises; as rock in the upper mantle cools, its density increases, and it sinks.
Example: when new magma is injected into a magma chamber, its density is lower than the cooler bulk of the magma chamber, so it rises, while the cooler magma in the chamber sinks.
Arthur Holmes realized as early as 1931 that thermal convection in the mantle drives the plate tectonics of the Earth
convection accounts for about 6X more heat transfer in the mantle than conduction (this ratio of convection to conduction is called the Nusselt number)
advection: the transfer of heat by motion of rocks
e.g., cold rock is carried deeper in subduction zones
e.g., hot rock is carried upward in dikes.
PRESSURE
P=pqz; p is density, g the acceleration of gravity, and z depth
material density (g/cm3) density (kg/m3)
water 1 1000
sedimentary rock 2.7 2700
mafic rock 3.0 3000
upper mantle 3.3 3300
e.g., what is the pressure at the base of a 10 km column of ultramafic rock?
= 3,300 kg/m3 * 10 m/s2 * 10,000 m
= ~3.3E8 kg m s-2 m-3 m
= 3.3E8 N/m2 (recall that N = kg m s-2)
= 3.3E8 Pa (recall that Pa = N/m2)
= 330 MPa (=3 kbar)
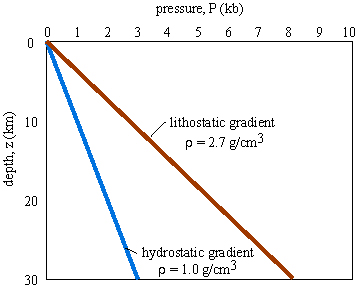
hydrostatic pressure: the pressure caused by a column of water. In the upper crust of the Earth, there are enough fractures, cracks, and porosity that the fluid within these voids is under hydrostatic pressure.
hydrostatic pressure gradient: the (vertical) gradient in hydrostatic pressure
lithostatic pressure: the pressure caused by a column of rock
lithostatic pressure gradient: the (vertical) gradient in lithostatic pressure
nonhydrostatic, tectonic stresses have little effect on sum pressure.Typical tectonic stresses in the Earth are <100 MPa, such that a lithostatic pressure of 300 MPa (i.e., ~10 km depth) is affected by less than 30 MPa.
uplift: increase in elevation
exhumation: decrease in vertical separation between a rock and the surface
Rates of Exhumation process dz/dt (mm/a or km/Ma)
burial by sedimentation 0.01 Ś> 1
''burial'' by subduction 10 Ś> 100
erosional exhumation -0.1 Ś> -10
tectonic exhumation -10 Ś> -100
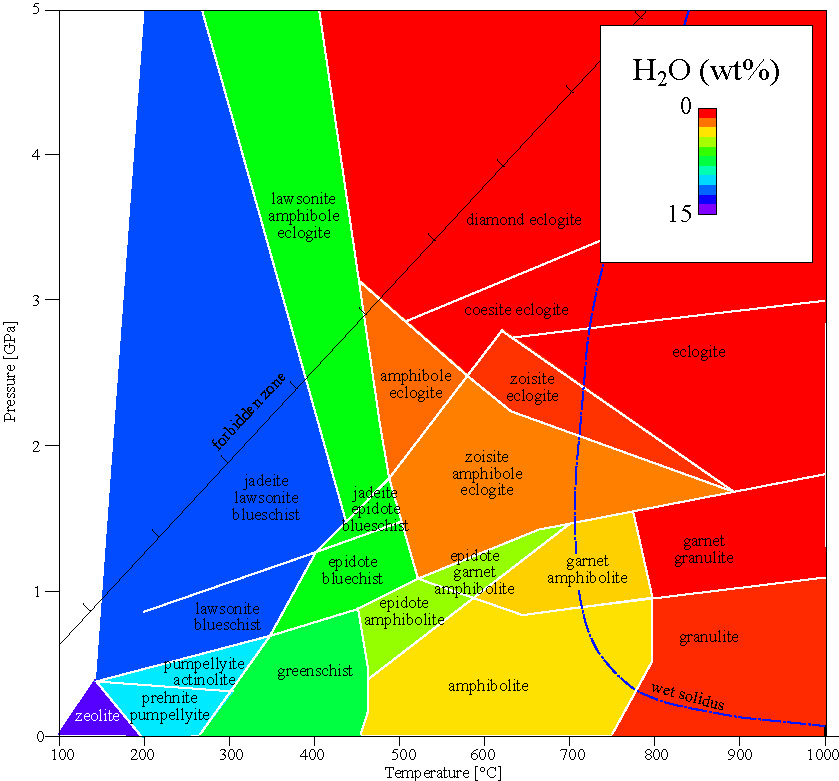
METAMORPHIC FACIES
facies minerals notes
basalt and gabbro protoliths plagioclase NaAlSi3O8-CaAl2Si2O8 + pyroxene CaSiO3-MgSiO3-FeSiO3 .
zeolite facies common zeolites: laumontite, wairakite, analcime (Na,Ca)AlSiO(OH)x zeolites: channel structure makes them useful for engineering
common textures: in cracks, alteration of feldspar or glass
greenschist facies albite + epidote + actinolite + chlorite + quartz
albite NaAlSi3O8 takes up Na, Al, Si
epidote CaAlSiO(OH)x takes up Ca
actinolite Ca2(Fe,Mg)5Si8O22(OH)2 takes up Fe, Mg
chlorite (Mg,Fe)(AlSi)O(OH)x .
albite-epidote amphibolite facies albite + epidote + hornblende + quartz
albite NaAlSi3O8 takes up Na, Al, Si
epidote CaAlSiO(OH)x takes up Ca
hornblende (K,Na)(Ca,Fe,Mg)(AlSi)O22(OH)2 modal proportion of albite decreases, Na enters amphibole
modal proportion of chlorite decreases, Fe + Mg enters amphibole
amphibolite facies plagioclase + hornblende + quartz epidote replaced by intermediate plagioclase
remainder of elements in hornblende
granulite facies orthopyroxene + clinopyroxene + plagioclase + quartz must have 2 pyroxenes; can also have hornblende or garnet
blueschist facies glaucophane Na2Mg3Al2Si8O22(OH)2
lawsonite or epidote CaAlSiO(OH)x high P, low T
eclogite facies omphacite (Na,Al,Mg,Fe)Si2O6
garnet (Ca,Mg,Fe,Mn)3Al2Si3O12 high P, high T
coesite-eclogite facies coesite SiO2
diamond C
majorite Mg4Si4O12 - extreme pressure
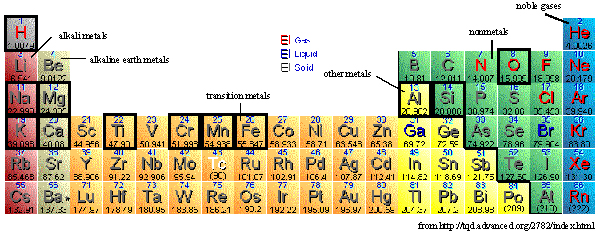
Link Between Elements and Minerals
Nine common mineral-forming elements element mineral
Si silicates
Al most silicates
Fe + Mg ferro-magnesian minerals
Ca feldspar or epidote
Na feldspar or jadeite
K mica or K-feldspar
O most minerals
H hydrous minerals
Next most common mineral-forming elements element mineral
P + Y + Ce apatite, xenotime, monazite
S sulfide minerals
Ti sphene, rutile, and ilmenite
Cr amphibole, chromite
Mn amphibole
Co + Ni olivine
Zn + Li staurolite
Sr carbonate minerals, feldspars
Zr + Hf + U + Pb zircon
KINETICS
While much of our interpretation of metamorphic rocks revolves around the assumption of equilibrium among the various phases, there is abundant evidence of disequilibrium in rocks, including:
zoning in minerals
preservation of pre-metamorphic minerals
incomplete retrogression
diamond rings
TRANSFORMATIONS
Phase transformations (reactions) consist of two parts: nucleation and then growth. Kinetic experiments have revealed a great deal of information about nucleation mechanism, nucleation rate, growth mechanism, and growth rate. Neoblasts can be observed once they have grown to a sufficient size.
The growth of one phase into another depends on factors including the Gibbs free energy change of the reaction, interfacial free energy, strain free energy, and temperature. Growth mechanisms can be studied in considerable detail with transmission-electron microscopy and optical ones.
As a specific example of kinetic experiments, let''s consider the transformation of laumontite (a zeolite) to wairakite (another zeolite) + quartz + H2O
Different textures develop at low and high temperatures