| Binary Phase Diagrams |
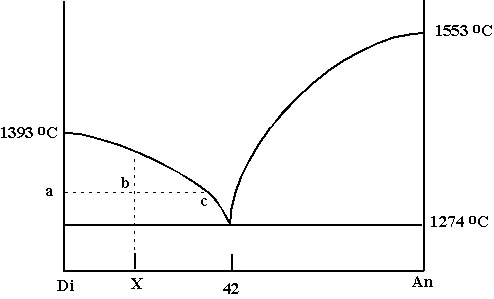
Di-An |
X-T phase diagram |
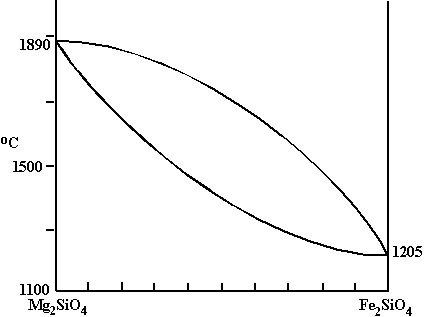
Olivine system |
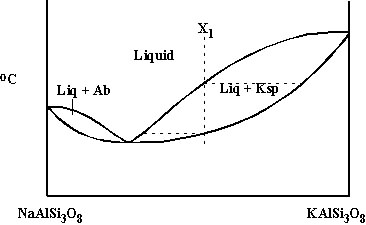
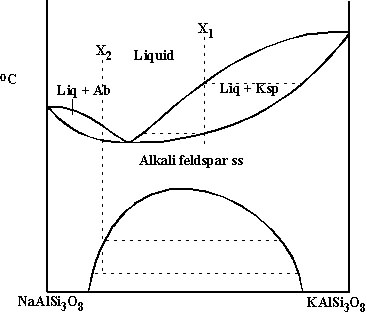
Solid solution with a minimus: alkali feldspars NaAlSi3O8-KAlSi3O8 (Ab-Ksp) |
Consider NaAlSi3O8-KAlSi3O8 again |
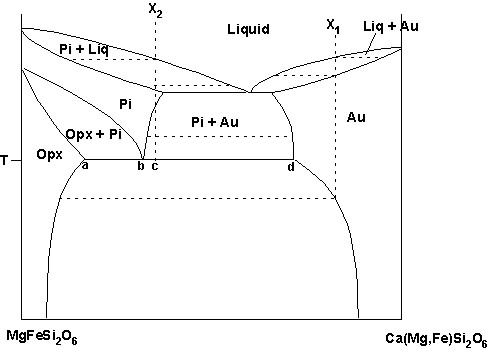
Pyroxene system: MgFeSi2O6-Ca(Mg,Fe)Si2O6 |
C http://ijolite.geology.uiuc.edu/08SprgClass/geo436/lectures.html
http://ijolite.geology.uiuc.edu/08SprgClass/geo436/436%20lectures/L6-Phase2.html
I. Binary System with 2 phases and no solid solution
A. Diopside-Anorthite system: equilibrium melting
- Figure Di-An
-Lever Rule: starting composition X = 18%An and 82% Di: ab/ac = % liquid at temperature T; bc/ac = % Di crystals at T; At c, liquid is 38% An and 62% Di (thus % liquid at c = 18/38 = 47% / % Di at T = 20/38 = 52%); at eutectic, 42% An, 58% Di.
-Trace this procedure in reverse for melting.
-This only applies if crystals remain in contact with liquid, and compositions continually change => equilibrium crystallization.
B. Di-An system: fractional crystallization
-Def: crystals are removed from magma as they form (sink or float).
-Liquid composition becomes new starting composition.
-Liquid becomes progressively depleted in phase that is crystallizing and enriched in phase that remains in the melt.
-Perfect fractional crystallization and equilibrium crystallization are extremes. In nature, all gradations in between.
C. What does this phase diagram tell us about igneous rocks?
-Lowest melting T occurs at eutectic.
-Most igneous rocks have compositions near eutectics => there is just enough thermal energy in Earth to melt the lowest-T compositions: basalts ~ Di-An eutectic; granites ~Ab-Q eutectic (system is similar to Di-An)
-Textures: start with An-rich basaltic melt: first crystals are An - plenty of room & time to grow=> large crystals = phenocrysts. Texture is called porphyritic; if magma on liquidus erupts, liquid quenches to glass surrounding phenocrysts = vitrophyric texture; at eutectic, both crystallize at same time, have to accommodate each other''s growth => eutectic intergrowths (basalt: ophitic texture = plag laths surrounded by single pyroxene grains / granite: graphic granite = quartz grains in large single crystals of alkali feldspar)
D. System with binary compound
-Def: phase with composition intermediate between end members: Ne + 2Q <> Ab
-Some compounds melt congruently = to a liquid of their own composition; others incongruently = melts to form solid + liquid where both have different composition from compound.
-Congruent melting - Ne-Q system ( ab melts congruently at 1118 oC => / ivides diagram into adjoining binary eutectic diagrams /each works like Di-An); congruent Ab divides diagram => 2 eutectics ~granite & nepheline syenite ; consider 2 liquids, compositions close but on opposite sides of albite: on Q-rich side, eventually SiO2 crystallizes; on other side, Ne. This accounts for division of igneous rocks into 2 groups (oversaturated = containing Q /undersaturated = containing feldspathoids ); Q and Ne have high-T polymorphs: Si ones have no solid solution with Ab, Ne ones do (more complicated).
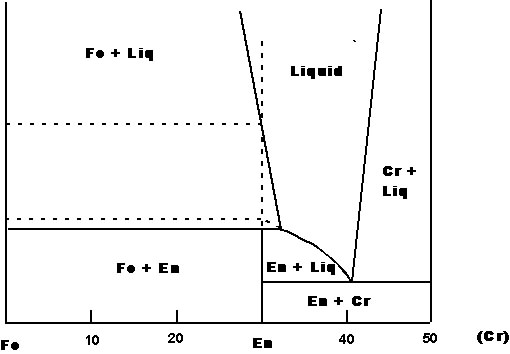
-Incongruent melting - Fo-Q system has a binary compound = En: X-T phase diagram (consider heating En: T rises to T1 => En melts to Fo+liq of composition P = peritectic: 3 phases (Fo, En, liq) => invariant point/T doesn''t change until all En is melted. Then T rises, Fo begins to melt & liq changes along Fo liquidus/at T2, all Fo melts, and liquid has composition of En/ consider cooling x1: Fo forms first. As T decreases, liq changes along Fo liquidus to P. Then Fo reacts with liq to form En. T stays unchanged until all liq is used up. Then system = solid Fo + En/ consider cooling x2: Fo forms first, liq changes along line to P, then reacts, as before. This time, Fo is used up first leaving En + liq. As T drops, liq changes along En liquidus to eutectic. Then En + Cr form until all SiO2 is used up. Solid En + Cr/ consider cooling x3: T drops until it intersects En liquidus and En forms without intermediate Fo/ what does this phase diagram tell us about igneous rocks?/liquids of peritectic composition ~ another common igneous rock group = continental flood basalts/melting of Cr + En yields eutectic melt. But melting of Fo + En begins at peritectic composition/note also that if this liquid is later cooled, Cr can form, ev en though there was none in the source)
Binary Phase Diagrams
I. Binary System with complete solid solution
A. Definition
-In all previous diagrams, solid phases had single composition, and only liquid composition changed.
-For many minerals, changing the amount of one component in the system changes the solid composition: solid solution: feldspars; olivines; amphiboles; pyroxenes
B. Olivine system: Fo-Fa, Mg2SiO4-Fe2SiO4
-Plot liquidus: no eutectic; liquid composition changes continuously; tmelt is highest for Fo, lowest for Fa, and intermediate for all mixtures
-Plot solidus: not a horizontal line. Solid composition also changes during melting or crystallization.
A. Notes
-System is never isobarically invariant
-Liquidus & solidus are univariant lines.
-The constant equilibration between solid & liquid with falling T is a continuous reaction.
-Contrast with behavior of a system with a peritectic: 2 solids + liquid = 3 phases = invariant. Peritectic is an inflection point in the liquids => called a discontinuous reaction.
B. Zoning
-If reaction between liquid and core of crystal is slow, center may retain a more Mg-rich composition, surrounded by successively more Fe-rich mantles.
-In olivine reactions are relatively uniform. Isolated tetrahedra => fairly easy to homogenize the structure as liquid composition changes.
-In pyroxenes, single chains allow easier diffusion || chain, so zoning may be directional.
-In plag, frameworks require diffusion of Na, Ca, Al, Si => plag usually strongly zoned.
C. Fractionation
-Consider solid Fa80. Raise T to solidus, first liquid ~Fa95.
-If this liquid is separated from the residual solid, then cooled, liquid composition enriched in Fa.
-Continual fractionation could yield pure Fa.
D. Bowen''s Reaction Series
-Plag side is labeled continuous: plag system is analogous to olivine system.
-Other side is labeled discontinuous: ex: in Fo-Q system, En forms discontinuously at peritecti; similarly for change to amphibole, then biotite.
E. Solid solution with a minimus: alkali feldspars NaAlSi3O8-KAlSi3O8 (Ab-Ksp)
-Composition with ~Ksp28 has minimum melting T.
-This divides diagram into 2 solid solution loops, one Ksp-rich and one Ab-rich: equilibrium crystallization works the same as for previous type of system, except that right of minimum, compositions become more Ab-rich, and left of minimum, compositions become more Ksp-rich; fractional crystallization pushes liquid composition toward the minimum, from either side.
I. Partial solid solution
A. Some minerals form complete solid solution at high T, but at lower T separate into 2 phases with distinct compositions.
-The line that divides single phase from 2 phases = solvus.
-Examples: alkali feldspars; clino- and orthopyroxenes; Ca-rich and Ca-poor amphiboles;Ilmenite and hematite
-Chalcopyrite and pyrrhotite
-This is a solid-state reaction (doesn''t involve liquid)
B. Consider NaAlSi3O8-KAlSi3O8 again. What happens at lower T?
-Complete solid solution occurs immediately below minimum.
-However, at lower T, alkali feldspar separates into 2 separate phases Ab and Ksp.
-Alkali feldspar ss cools until it intersects solvus: then Ksp exsolves from Ab = forms separate crystals within Ab host (individual crystals often stringy or wispy: exsolution lamellae); Ab phase becomes more sodic with further cooling; Ksp phase more potassic; relative amounts of Ab and Ksp at any T can be found from lever rule.
C. Pyroxene system: MgFeSi2O6-Ca(Mg,Fe)Si2O6
-Figure is pseudobinary (constant Mg/(Mg+Fe))
-It shows 3 solvi