| Phase Diagrams with More Than Two Components |
Add 3rd dimension: temperature. Add 3rd dimension: temperature |
System nepheline-albite-sodium silicate |
System nepheline-silica-diopside - congruent melting - binary compound |
System forsterite-silica-anorthite - binary compound - incongruent melting |
System diopside-anorthite-albite |
System albite-anorthite-diopside-forsterite |
See http://plate-tectonic.narod.ru/petrographyigneouslinks.html
C http://ijolite.geology.uiuc.edu/08SprgClass/geo436/lectures.html
http://ijolite.geology.uiuc.edu/08SprgClass/geo436/436%20lectures/L8-Phase4.html
I. Ternary systems: 3 components
A. Representation by an equilateral triangle
-Vertex A = 100% A, 0% B and C
-A ranges from 0% along BC to 100% at A
-Any point on AB = mixture of A and B, no C
-Any point in the interior of the triangle = mixture of all 3
-Note: this holds even if the triangle is not equilateral.
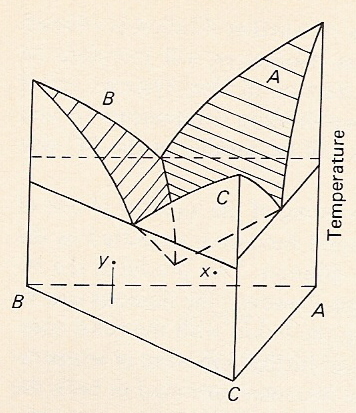
B. Lever rule applies
-Along side BC, A=0: let X be along side BC. Then %B = XC/BC ; %C = BX/BC
-If A is added, ratio B and C doesn''t change => composition lies along line XA
-At Y along XA, composition is: %A = XY/XA; %(B+C) = YA/XA (but B/C = XC/BX, so %B = (XC/BC)*(YA/XA)/%C = (BX/BC)*(YA/XA)
-Diagram this out on paper to confirm!
C. Add 3rd dimension: temperature
-Simplest ternary diagram: each side represents a binary eutectic system: liquidus lines become surfaces ; eutectic points become cotectic lines; a ternary eutectic forms where the 3 liquidus surfaces intersect; arrows show direction of decreasing temperature
II. Ternary systems without solid solution
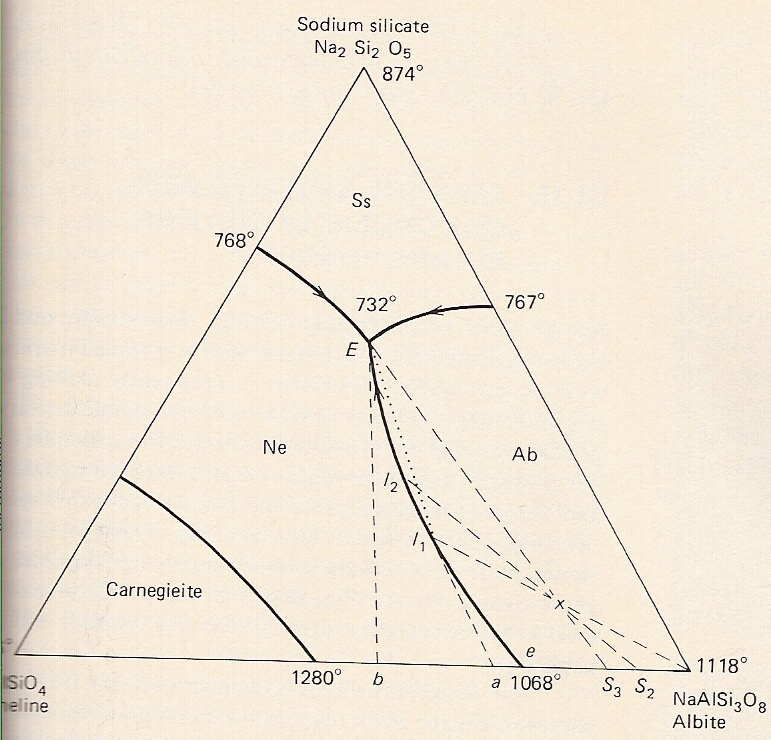
A. System nepheline-albite-sodium silicate
-Components Ne-Ab-Ss
-Binary eutectics along each side => 3 cotectic lines.
-Adding the 3rd component to each side lowers the temperature below the binary eutectic. 3 cotectic lines intersect at ternary eutectic point = lowest melting temperature
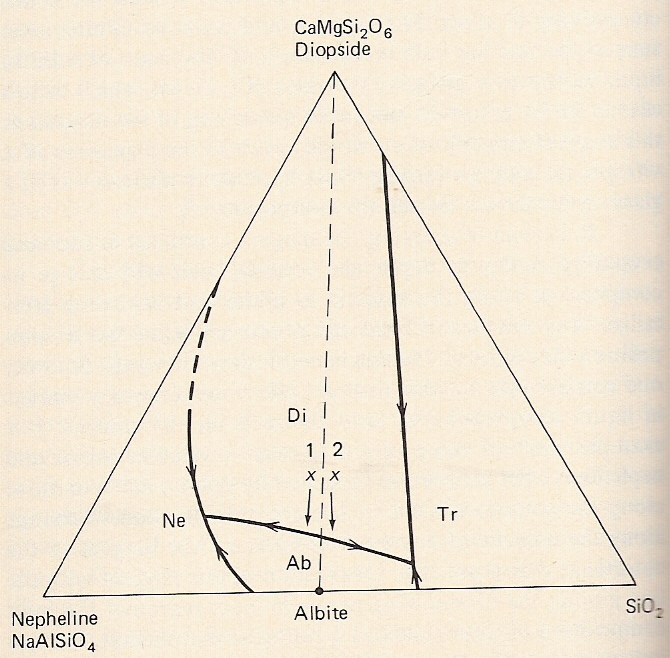
B. System nepheline-silica-diopside - congruent melting - binary compound.
-Components Ne-Tr-Di: albite forms between Ne and Tr; line joining Ab and Di separates equilateral triangle into 2 separate triangular diagrams.
-Ab-Di represents a thermal divide
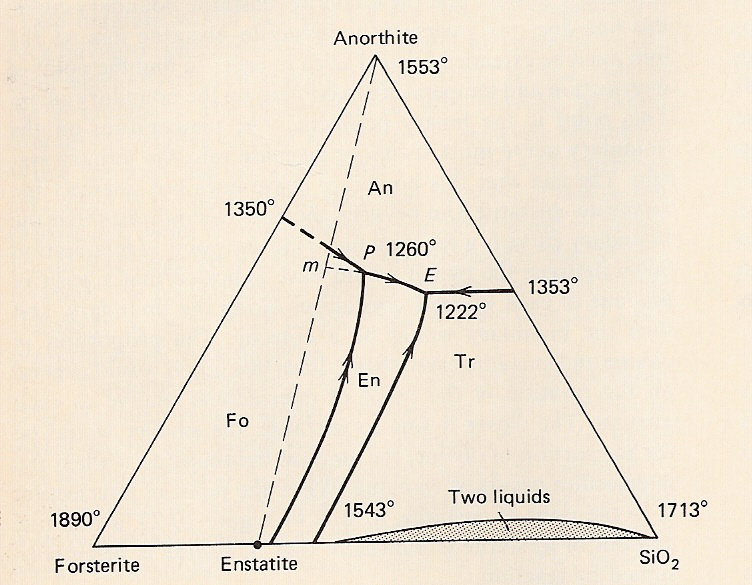
C. System forsterite-silica-anorthite - binary compound - incongruent melting
-Components Fo-Tr-An
-Recall enstatite melts incongruently to Fo + liquid
-Peritectic point in binary diagram becomes a reaction curve in ternary: in 3D diagram, cotectic lines ~ valleys; reaction curve ~ inflection point (change in slope)
III. Ternary systems with solid solution
A. One or more sides represent solid solution binary systems (no binary eutectic)
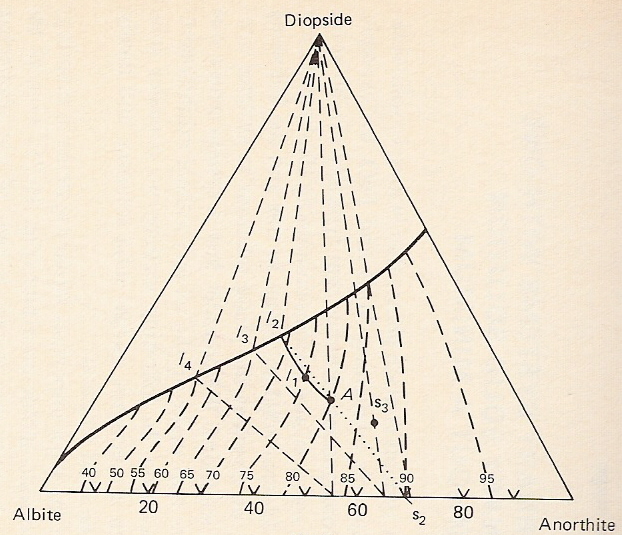
B. System diopside-anorthite-albite
-Components Di-An-Ab
-Di-An and Di-Ab sides represent binary eutectic systems - generates cotectic line in ternary diagram
-Ab-An side is solid solution loop - no minimum.
-Thus there is no ternary eutectic. One cotectic line descends continuously from Di-An eutectic temperature to Di-Ab eutectic temperature.
-At any point along cotectic, Di crystallizes along with plagioclase of intermediate composition.
IV. Quaternary systems
A. Models real systems very well, but graphical representation becomes difficult (4D)
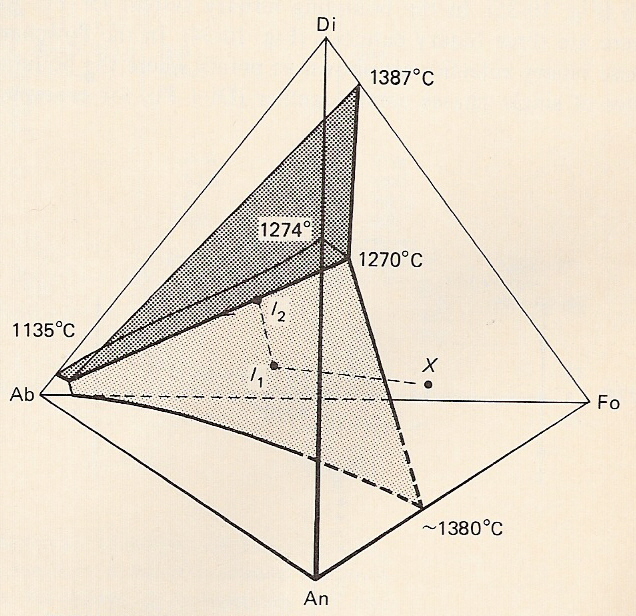
B. System albite-anorthite-diopside-forsterite
-Figure represents system as a tetrahedron
-Fo-Di-An and Ab-Fo-Di sides are simple ternary eutectics.
-Ab-An-Di and Ab-An-F o sides have no eutectic because of solid solution between Ab and An.
-Note: since 3 dimensions represent 4 components, T and P can''t be shown. Tetrahedral diagrams are isobaric and isothermal